A compound of formula XCl3 reacts with aqueous AgNO3 to yield solid AgCl according to the following equation: When a solution containing 0.634 g of XCl3 was allowed to react with an excess of aqueous AgNO3, 1.68 g of solid AgCl was formed. What is the identity of the atom X?
Ch.3 - Mass Relationships in Chemical Reactions

Chapter 3, Problem 117b
A copper wire having a mass of 2.196 g was allowed to react with an excess of sulfur. The excess sulfur was then burned, yielding SO2 gas. The mass of the copper sulfide produced was 2.748 g. (b) What is its empirical formula?
 Verified step by step guidance
Verified step by step guidance1
Determine the mass of sulfur that reacted by subtracting the mass of copper from the mass of copper sulfide: \( \text{mass of sulfur} = \text{mass of copper sulfide} - \text{mass of copper} \).
Convert the mass of copper to moles using its molar mass: \( \text{moles of copper} = \frac{\text{mass of copper}}{\text{molar mass of copper}} \).
Convert the mass of sulfur to moles using its molar mass: \( \text{moles of sulfur} = \frac{\text{mass of sulfur}}{\text{molar mass of sulfur}} \).
Determine the simplest whole number ratio of moles of copper to moles of sulfur by dividing each by the smallest number of moles calculated.
Use the mole ratio to write the empirical formula of the copper sulfide compound.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Empirical Formula
The empirical formula of a compound represents the simplest whole-number ratio of the elements present in that compound. It is determined by calculating the moles of each element and then dividing by the smallest number of moles to find the simplest ratio. This formula does not provide information about the actual number of atoms in a molecule but rather the relative proportions of each element.
Recommended video:
Guided course

Empirical vs Molecular Formula
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between the reactants and products in a chemical reaction. It involves using balanced chemical equations to calculate the amounts of substances consumed and produced. In this context, stoichiometry helps determine the moles of copper and sulfur that reacted to form copper sulfide, which is essential for finding the empirical formula.
Recommended video:
Guided course

Stoichiometry Concept
Mass Conservation
The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. This principle is crucial for calculating the mass of reactants and products. In this problem, the initial mass of copper and the mass of sulfur used can be related to the mass of the copper sulfide produced, allowing for the determination of the empirical formula based on the mass changes during the reaction.
Recommended video:
Guided course
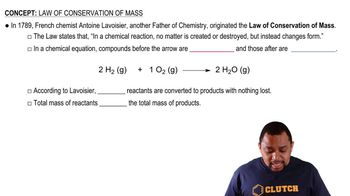
Law of Conservation of Mass
Related Practice
Textbook Question
Textbook Question
When eaten, dietary carbohydrates are digested to yield glu-cose (C6H12O6), which is then metabolized to yield carbon dioxide and water: Balance the equation, and calculate both the mass in grams and the volume in liters of the CO2 produced from 66.3 g of glucose, assuming that 1 mol of CO2 has a volume of 25.4 L at normal body temperature.
Textbook Question
A copper wire having a mass of 2.196 g was allowed to react with an excess of sulfur. The excess sulfur was then burned, yielding SO2 gas. The mass of the copper sulfide produced was 2.748 g. (a) What is the percent composition of copper sulfide?
Textbook Question
Element X, a member of group 5A, forms two chlorides, XCl3 and XCl5. Reaction of an excess of Cl2 with 8.729 g of XCl3 yields 13.233 g of XCl5. What is the atomic weight and the identity of the element X?
1
views
Textbook Question
Ammonium nitrate, a potential ingredient of terrorist bombs, can be made nonexplosive by addition of diammo-nium hydrogen phosphate, (NH4)2HPO4. Analysis of such a NH4NO3 - (NH4)2HPO4 mixture showed the mass percent of nitrogen to be 30.43%. What is the mass ratio of the two components in the mixture?
