Textbook Question
The vapor pressure of CCl3F at 300 K is 856 torr. If 11.5 g of CCl3F is enclosed in a 1.0-L container, will any liquid be present? If so, what mass of liquid?

 Verified step by step guidance
Verified step by step guidance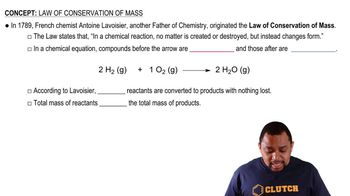


The vapor pressure of CCl3F at 300 K is 856 torr. If 11.5 g of CCl3F is enclosed in a 1.0-L container, will any liquid be present? If so, what mass of liquid?
A sample of steam with a mass of 0.552 g and at a temperature of 100 °C condenses into an insulated container holding 4.25 g of water at 5.0 °C. Assuming that no heat is lost to the surroundings, what is the final temperature of the mixture?