Based on the following reaction profile, how many intermediates are formed in the reaction A¡C? How many transition states are there? Which step, A¡B or B¡C, is the faster? For the reaction A¡C, is ΔE positive, negative, or zero? [Section 14.6]
Ch.14 - Chemical Kinetics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 14, Problem 11b
The following graph shows two different reaction pathways for the same overall reaction at the same temperature. Is each of the following statements true or false? (b) For both paths, the rate of the reverse reaction is slower than the rate of the forward reaction.

 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the forward and reverse reactions on the graph. The forward reaction progresses from left to right, while the reverse reaction progresses from right to left.
Step 2: Observe the energy barriers (activation energies) for both the forward and reverse reactions. The activation energy is the peak of the curve minus the energy of the reactants for the forward reaction, and the peak of the curve minus the energy of the products for the reverse reaction.
Step 3: Compare the activation energies for the forward and reverse reactions for both pathways. The pathway with the lower activation energy will have a faster reaction rate.
Step 4: Determine if the activation energy for the reverse reaction is higher than that for the forward reaction in both pathways. If it is, then the rate of the reverse reaction is slower than the rate of the forward reaction.
Step 5: Conclude whether the statement 'For both paths, the rate of the reverse reaction is slower than the rate of the forward reaction' is true or false based on the comparison of activation energies.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Pathways
Reaction pathways illustrate the energy changes that occur during a chemical reaction. They depict the energy of reactants, products, and the transition states, which are the highest energy points along the pathway. Understanding these pathways helps in analyzing how different conditions or catalysts can affect the rate and mechanism of a reaction.
Recommended video:
Guided course
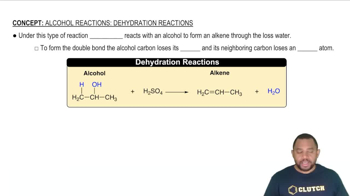
Alcohol Reactions: Dehydration Reactions
Forward and Reverse Reactions
In a chemical reaction, the forward reaction refers to the process where reactants are converted into products, while the reverse reaction is the conversion of products back into reactants. The rates of these reactions can differ based on the energy barriers depicted in the reaction pathway, influencing the overall dynamics of the reaction system.
Recommended video:
Guided course

Reversible Changes in Matter
Activation Energy
Activation energy is the minimum energy required for a reaction to occur. It is represented by the height of the energy barrier between reactants and the transition state in a reaction pathway. A higher activation energy typically results in a slower reaction rate, which is crucial for determining whether the forward or reverse reaction is favored under specific conditions.
Recommended video:
Guided course
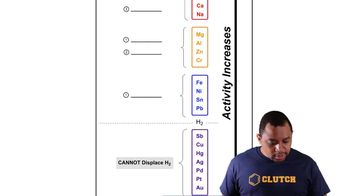
Activity Series Chart
Related Practice
Textbook Question
Textbook Question
The accompanying graph shows plots of ln k versus 1>T for two different reactions. The plots have been extrapolated to the y-intercepts. Which reaction (red or blue) has (a) the larger value for Ea,
Textbook Question
The accompanying graph shows plots of ln k versus 1>T for two different reactions. The plots have been extrapolated to the y-intercepts. Which reaction (red or blue) has (b) the larger value for the frequency factor, A? [Section 14.5]
2
views
Textbook Question
Consider the diagram that follows, which represents two steps in an overall reaction. The red spheres are oxygen, the blue ones nitrogen, and the green ones fluorine. (d) Write the rate law for the overall reaction if the first step is the slow, rate-determining step. [Section 14.6]
