Multiple Choice
Which element possesses a -2 charge when it combines with other elements?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: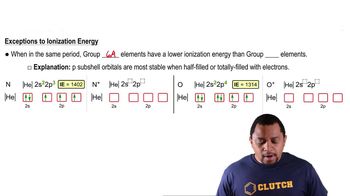


 2:15m
2:15mMaster Periodic Table: Charges with a bite sized video explanation from Jules
Start learning