The distance from the sun to Earth is 1.496×108 km. How long does it take light to travel from the sun to Earth?

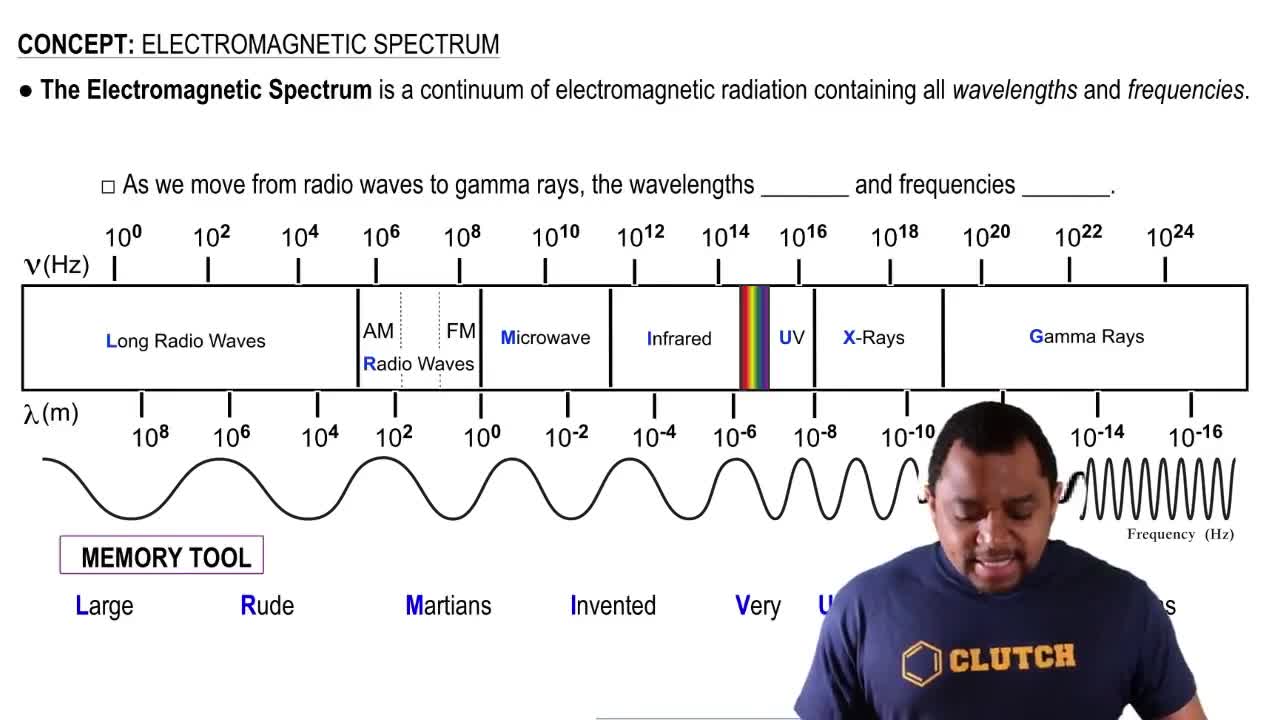
List these types of electromagnetic radiation in order of (ii) increasing energy per photon.
a. visible light
b. radio waves
c. X-ray
d. ultraviolet radiation
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Electromagnetic Spectrum
Photon Energy
Order of Increasing Energy

The nearest star to our sun is Proxima Centauri, at a distance of 4.3 light-years from the sun. A light-year is the distance that light travels in one year (365 days). How far away, in km, is Proxima Centauri from the sun?
List these types of electromagnetic radiation in order of (i) increasing frequency and (ii) decreasing energy per photon.
a. gamma rays
b. ultraviolet radiation
c. infrared radiation
d. microwaves
Calculate the frequency of each wavelength of electromagnetic radiation: a. 632.8 nm (wavelength of red light from helium–neon laser) b. 503 nm (wavelength of maximum solar radiation) c. 0.052 nm (wavelength contained in medical X-rays)
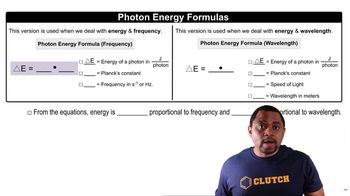
Calculate the energy of a photon of each wavelength and state what part of the electromagnetic spectrum is associated with the wavelength.
a. 105 nm
b. 715 nm
c. 2.52 cm
