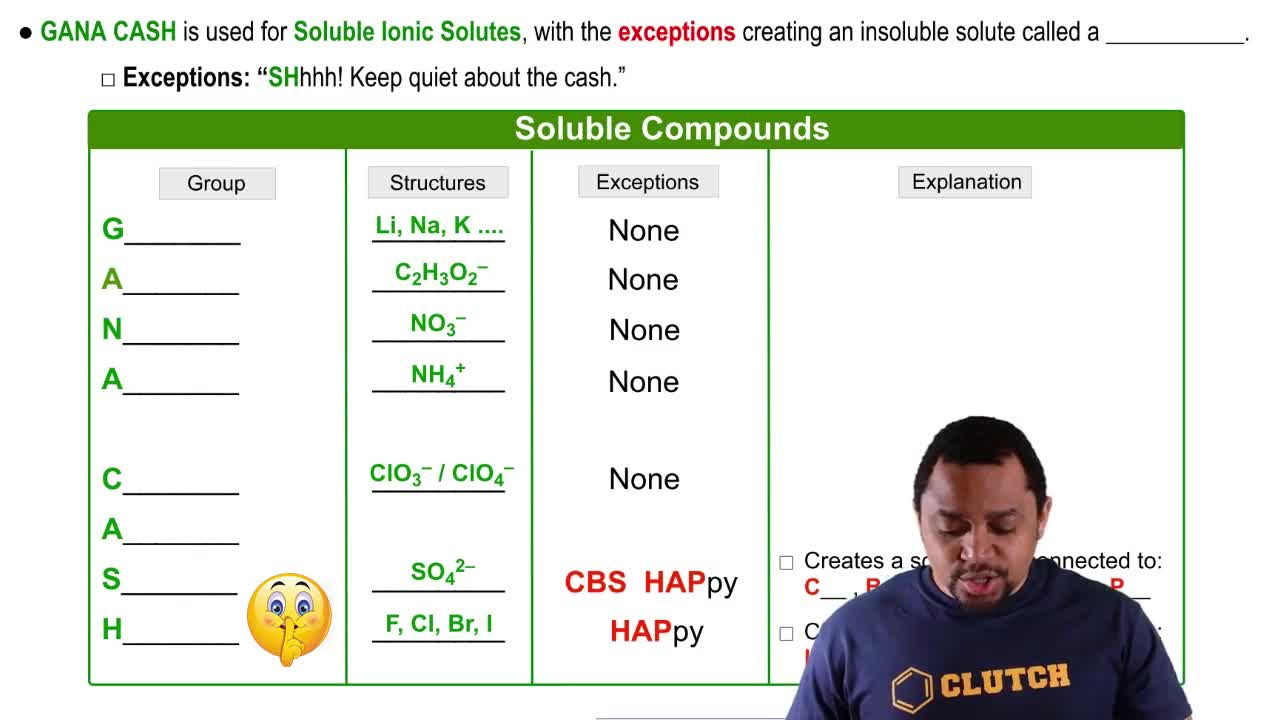
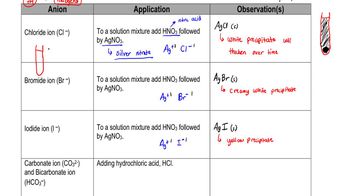
Solubility and Ionic Compounds
Ionic compounds like NaCl, KCl, and MgCl2 are generally soluble in water, but their solubility can vary. Understanding the solubility rules helps predict how these compounds will behave in solution. For instance, while NaCl and KCl are highly soluble, MgCl2 has a different solubility profile due to the presence of magnesium, which can affect the ionic interactions in water.

 Verified step by step guidance
Verified step by step guidance