For each of the following compounds, write a balanced thermochemical equation depicting the formation of one mole of the compound from its elements in their standard states and then look up ΔH°f for each substance in Appendix C. (a) NO2(g) (b) SO3(g) (c) NaBr(s) (d) Pb(NO3)2(s).
Ch.5 - Thermochemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 5, Problem 68
(a) Why does the standard enthalpy of formation of both the very reactive fluorine (F2) and the almost inert gas nitrogen (N2) both read zero?
 Verified step by step guidance
Verified step by step guidance1
Understand that the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance is formed from its elements in their standard states.
Recognize that the standard state of an element is its most stable form at 1 atmosphere of pressure and a specified temperature, usually 25°C (298 K).
Note that for any element in its standard state, the standard enthalpy of formation is defined to be zero because no formation reaction is needed to produce the element from itself.
Identify that both fluorine (F_2) and nitrogen (N_2) are in their most stable forms as diatomic molecules under standard conditions.
Conclude that since F_2 and N_2 are in their standard states, their standard enthalpies of formation are zero by definition.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
55sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Standard Enthalpy of Formation
The standard enthalpy of formation is defined as the change in enthalpy when one mole of a compound is formed from its elements in their standard states. For elements in their standard state, such as F<sub>2</sub> and N<sub>2</sub>, the standard enthalpy of formation is assigned a value of zero. This serves as a reference point for calculating the enthalpy changes of reactions involving these elements.
Recommended video:
Guided course

Enthalpy of Formation
Standard State
The standard state of a substance is its physical state at a defined set of conditions, typically 1 bar of pressure and a specified temperature, usually 25°C. For diatomic gases like F<sub>2</sub> and N<sub>2</sub>, their standard state is the gaseous form at these conditions. Understanding standard states is crucial for interpreting thermodynamic data and calculating reaction enthalpies.
Recommended video:
Guided course

Standard Reduction Potentials
Reactivity and Stability of Elements
Reactivity refers to how readily a substance undergoes chemical reactions, while stability indicates how likely it is to remain unchanged. Fluorine (F<sub>2</sub>) is highly reactive due to its strong electronegativity and tendency to form bonds, whereas nitrogen (N<sub>2</sub>) is relatively inert due to the strong triple bond between nitrogen atoms. Despite their differences in reactivity, both elements have a standard enthalpy of formation of zero because they are in their elemental forms.
Recommended video:
Guided course
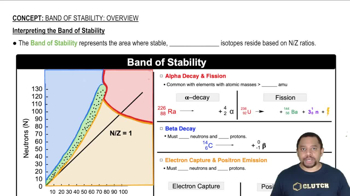
Intepreting the Band of Stability
Related Practice
Textbook Question
Textbook Question
From the enthalpies of reaction H2(g) + F2(g) → 2 HF(g) ΔH = -537 kJ C(s) + 2 F2(g) → CF4(g) ΔH = -680 kJ 2 C(s) + 2 H2(g) → C2H4(g) ΔH = +52.3 kJ Calculate H for the reaction of ethylene with F2: C2H4(g) + 6 F2(g) → 2 CF4(g) + 4 HF(g)
Textbook Question
Given the data N2(g) + O2(g) → 2 NO(g) ΔH = +180.7 kJ 2 NO(g) + O2(g) → 2 NO2(g) ΔH = -113.1 kJ 2 N2O(g) → 2 N2(g) + O2(g) ΔH = -163.2 kJ use Hess's law to calculate ΔH for the reaction N2O(g) + NO2(g) → 3 NO(g)
Textbook Question
(c) What is meant by the term standard enthalpy of formation?
Textbook Question
Write balanced equations that describe the formation of the following compounds from elements in their standard states, and then look up the standard enthalpy of formation for each substance in Appendix C: (a) CH3OH(l)
