Based on their compositions and structures and on conjugate acid–base relationships, select the stronger base in each of the following pairs: (b) BrO- or BrO2-
Ch.16 - Acid-Base Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 16, Problem 91c
Indicate whether each of the following statements is true or false. For each statement that is false, correct the statement to make it true. (a) In general, the acidity of binary acids increases from left to right in a given row of the periodic table. (b) In a series of acids that have the same central atom, acid strength increases with the number of hydrogen atoms bonded to the central atom. (c) Hydrotelluric acid 1H2Te2 is a stronger acid than H2S because Te is more electronegative than S.
 Verified step by step guidance
Verified step by step guidance1
(a) True: In general, the acidity of binary acids increases from left to right across a period in the periodic table. This is because the electronegativity of the elements increases, which stabilizes the conjugate base, making the acid stronger.
(b) False: In a series of acids with the same central atom, acid strength increases with the number of oxygen atoms bonded to the central atom, not hydrogen atoms. For example, HClO4 is stronger than HClO3.
(c) False: Hydrotelluric acid (H2Te) is a stronger acid than H2S not because Te is more electronegative, but because Te is larger in size. The larger size of Te compared to S results in a weaker H-Te bond, making it easier to donate a proton.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Acidity Trends in the Periodic Table
The acidity of binary acids generally increases from left to right across a period in the periodic table. This trend is due to the increasing electronegativity of the central atom, which stabilizes the negative charge on the conjugate base after the acid donates a proton. As electronegativity increases, the bond between the hydrogen and the central atom becomes weaker, making it easier for the acid to release a proton.
Recommended video:
Guided course

Periodic Trends
Acid Strength and Hydrogen Atoms
In a series of acids with the same central atom, the strength of the acid does not necessarily increase with the number of hydrogen atoms bonded to the central atom. Instead, the strength is influenced by the ability of the central atom to stabilize the negative charge of the conjugate base after deprotonation. For example, in oxoacids, the presence of electronegative atoms can enhance acid strength, regardless of the number of hydrogen atoms.
Recommended video:
Guided course
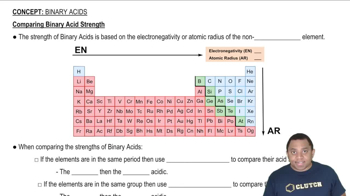
Comparing Binary Acid Strength
Electronegativity and Acid Strength
Electronegativity is a measure of an atom's ability to attract and hold onto electrons. In the context of acids, a more electronegative central atom can lead to stronger acids because it stabilizes the conjugate base formed after the acid donates a proton. However, the comparison of acid strength between different elements must consider other factors, such as atomic size and bond strength, rather than electronegativity alone.
Recommended video:
Guided course

Comparing Binary Acid Strength
Related Practice
Textbook Question
Textbook Question
Based on their compositions and structures and on conjugate acid–base relationships, select the stronger base in each of the following pairs: (b) PO43- or AsO43-
Textbook Question
Indicate whether each of the following statements is true or false. For each statement that is false, correct the statement to make it true. (a) Acid strength in a series of H¬A molecules increases with increasing size of A. (b) For acids of the same general structure but differing electronegativities of the central atoms, acid strength decreases with increasing electronegativity of the central atom. (c) The strongest acid known is HF because fluorine is the most electronegative element.
Textbook Question
The fluoride ion reacts with water to produce HF. (c) Is fluoride acting as a Lewis acid or as a Lewis base when reacting with water?
