The rate of disappearance of HCl was measured for the following reaction: CH3OH1aq2 + HCl1aq2¡CH3Cl1aq2 + H2O1l2 The following data were collected: Time (min) 3HCl 4 1M2 0.0 1.85 54.0 1.58 107.0 1.36 215.0 1.02 430.0 0.580 (a) Calculate the average rate of reaction, in M>s, for the time interval between each measurement.

The rate of disappearance of HCl was measured for the following reaction: CH3OH1aq2 + HCl1aq2¡CH3Cl1aq2 + H2O1l2 The following data were collected: Time (min) 3HCl 4 1M2 0.0 1.85 54.0 1.58 107.0 1.36 215.0 1.02 430.0 0.580 (d) Graph [HCl] versus time and determine the instantaneous rates in M>min and M>s at t = 75.0 min and t = 250 min.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Reaction Rate

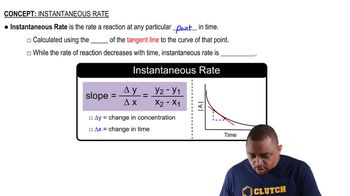
Instantaneous Rate
Graphing Concentration vs. Time

The rate of disappearance of HCl was measured for the following reaction: CH3OH1aq2 + HCl1aq2¡CH3Cl1aq2 + H2O1l2 The following data were collected: Time (min) 3HCl 4 1M2 0.0 1.85 54.0 1.58 107.0 1.36 215.0 1.02 430.0 0.580 (b) Calculate the average rate of reaction for the entire time for the data from t = 0.0 min to t = 430.0 min.
The rate of disappearance of HCl was measured for the following reaction: CH3OH1aq2 + HCl1aq2¡CH3Cl1aq2 + H2O1l2 The following data were collected: Time (min) 3HCl 4 1M2 0.0 1.85 54.0 1.58 107.0 1.36 215.0 1.02 430.0 0.580 (c) Which is greater, the average rate between t = 54.0 and t = 215.0 min, or between t = 107.0 and t = 430.0 min?
For each of the following gas-phase reactions, indicate how the rate of disappearance of each reactant is related to the rate of appearance of each product:
(a) H2O2(g) → H2(g) + O2(g)
(b) 2 N2O(g) → 2 N2(g) + O2(g)
For each of the following gas-phase reactions, indicate how the rate of disappearance of each reactant is related to the rate of appearance of each product: (c) N21g2 + 3 H21g2¡2 NH31g2
For each of the following gas-phase reactions, indicate how the rate of disappearance of each reactant is related to the rate of appearance of each product:
(d) C2H5NH2(g) → C2H4(g) + NH3(g)
