Given the following standard reduction potentials at 25 °C, (a) balance the equation for the reaction of H2MoO4 with elemental arsenic in acidic solution to give Mo3+ and H3AsO4 and (b) calculate E° for this reaction.
Ch.19 - Electrochemistry

Chapter 19, Problem 155b,c
The reaction of MnO4– with oxalic acid (H2C2O4) in acidic solution, yielding Mn2+ and CO2 gas, is widely used to determine the concentration of permanganate solutions. (b) Use the data in Appendix D to calculate E° for the reaction. (c) Show that the reaction goes to completion by calculating the values of ∆G° and K at 25 °C. (H2C2O4) in acidic solution, yielding Mn2+ and CO2 gas, is widely used to determine the concentration of permanganate solutions.
 Verified step by step guidance
Verified step by step guidance1
Identify the half-reactions involved in the redox process. For the given reaction, the reduction half-reaction is MnO4– + 8H+ + 5e– → Mn2+ + 4H2O, and the oxidation half-reaction is H2C2O4 → 2CO2 + 2H+ + 2e–.
Use the standard reduction potentials from Appendix D to find the E° values for each half-reaction. The standard reduction potential for MnO4– to Mn2+ is typically given, and you will need to reverse the sign for the oxidation of H2C2O4 to CO2.
Calculate the standard cell potential, E°, for the overall reaction by combining the half-reactions. Use the formula E° = E°(cathode) - E°(anode). Ensure that the number of electrons lost in the oxidation half-reaction equals the number gained in the reduction half-reaction by multiplying the half-reactions by appropriate coefficients.
Calculate the standard Gibbs free energy change, ΔG°, using the formula ΔG° = -nFE°, where n is the number of moles of electrons transferred in the balanced equation, F is the Faraday constant (approximately 96485 C/mol), and E° is the standard cell potential.
Determine the equilibrium constant, K, at 25 °C using the relationship between ΔG° and K: ΔG° = -RT ln K, where R is the universal gas constant (8.314 J/mol·K) and T is the temperature in Kelvin. Solve for K to show that the reaction goes to completion.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
13mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Standard Electrode Potential (E°)
The standard electrode potential (E°) is a measure of the tendency of a chemical species to be reduced, measured under standard conditions. It is crucial for predicting the direction of redox reactions and calculating the overall cell potential. In this context, E° values for the half-reactions involving MnO4– and oxalic acid will help determine the feasibility of the reaction.
Recommended video:
Guided course
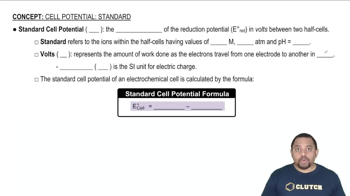
Standard Cell Potential
Gibbs Free Energy (∆G°)
Gibbs Free Energy (∆G°) is a thermodynamic quantity that indicates the spontaneity of a reaction at standard conditions. A negative ∆G° value suggests that a reaction is spontaneous and will proceed to completion. In this case, calculating ∆G° for the reaction will provide insight into whether the reaction between MnO4– and oxalic acid is thermodynamically favorable.
Recommended video:
Guided course
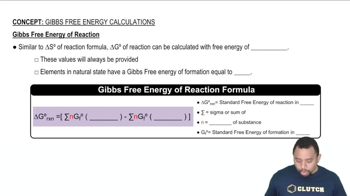
Gibbs Free Energy of Reactions
Equilibrium Constant (K)
The equilibrium constant (K) quantifies the ratio of the concentrations of products to reactants at equilibrium for a given reaction. It is related to Gibbs Free Energy by the equation ∆G° = -RT ln(K). A large K value indicates that products are favored at equilibrium, which, when calculated for the reaction, will confirm whether it goes to completion.
Recommended video:
Guided course

Equilibrium Constant K
Related Practice
Textbook Question
Textbook Question
The reaction of MnO4– with oxalic acid (H2C2O4) in acidic solution, yielding Mn2+ and CO2 gas, is widely used to determine the concentration of permanganate solutions. (a) Write a balanced net ionic equation for the reaction.
Textbook Question
The reaction of MnO4– with oxalic acid (H2C2O4) in acidic solution, yielding Mn2+ and CO2 gas, is widely used to determine the concentration of permanganate solutions. (d) A 1.200 g sample of sodium oxalate (Na2C2O4) is dissolved in dilute H2SO4 and then titrated with a KMnO4 solution. If 32.50 mL of the KMnO4 solution is required to reach the equivalence point, what is the molarity of the KMnO4 solution?
Textbook Question
A concentration cell has the same half-reactions at the anode and cathode, but a voltage results from different concentrations in the two electrode compartments.(b) A similar cell has 0.10 M Cu2+ in both compartments. When a stoichiometric amount of ethylenediamine (NH2CH2CH2NH2) is added to one compartment, the measured cell potential is 0.179 V. Calculate the formation constant Kf for the complex ion Cu(NH2CH2CH2CH2)22+. Assume there is no volume change.
