Each of the following pairs of elements will react to form a binary ionic compound. Write the formula of each compound formed, and give its name. (c) Lithium and nitrogen
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory

All textbooks McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 78b
Problem 78b
 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 78b
Problem 78bChapter 6, Problem 78b
Element X reacts with element Y to give a product containing X3+ ions and Y2-ions. (b) Is element Y likely to be a metal or a nonmetal? Explain.
 Verified step by step guidance
Verified step by step guidance1
Identify the charges on the ions formed in the reaction. Here, X forms X3+ ions and Y forms Y2- ions.
Recall that metals tend to lose electrons and form positively charged cations, while nonmetals tend to gain electrons and form negatively charged anions.
Analyze the charge on the Y ion. Since Y forms Y2- ions, it gains electrons.
Compare the behavior of Y with typical properties of metals and nonmetals. Gaining electrons is a characteristic of nonmetals.
Conclude that element Y is likely to be a nonmetal because it forms anions by gaining electrons.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
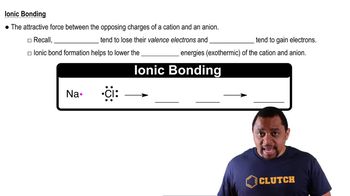
Ionic Bonding
Ionic bonding occurs when atoms transfer electrons, resulting in the formation of charged ions. In this case, element Y forms Y2- ions, indicating it has gained two electrons, which is characteristic of nonmetals. The attraction between the positively charged X3+ ions and negatively charged Y2- ions leads to the formation of an ionic compound.
Recommended video:
Guided course
Chemical Bonds
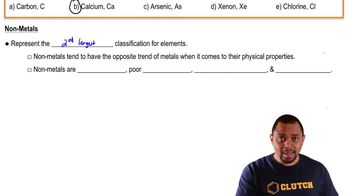
Metal vs. Nonmetal Properties
Metals are typically characterized by their ability to lose electrons easily, forming positive ions, while nonmetals tend to gain electrons to form negative ions. The formation of Y2- ions suggests that element Y is likely a nonmetal, as nonmetals are more electronegative and prefer to gain electrons to achieve a stable electron configuration.
Recommended video:
Guided course
Nonmetal Properties
Electronegativity
Electronegativity is a measure of an atom's ability to attract and hold onto electrons. Nonmetals generally have higher electronegativities compared to metals, which allows them to gain electrons and form anions. In this reaction, the formation of Y2- ions indicates that element Y has a high electronegativity, supporting the conclusion that it is a nonmetal.
Recommended video:
Guided course

Electronegativity Trends
Related Practice
Textbook Question
Textbook Question
Each of the following pairs of elements will react to form a binary ionic compound. Write the formula of each compound formed, and give its name. (d) Barium and flourine
Textbook Question
Element X reacts with element Y to give a product containing X3+ ions and Y2-ions. (a) Is element X likely to be a metal or a nonmetal? Explain.
Textbook Question
Element X reacts with element Y to give a product containing X3+ ions and Y2- ions. (c) What is the formula of the product?
Textbook Question
Element X reacts with element Y to give a product containing X3+ ions and Y2-ions. (d) In what groups of the periodic table are elements X and Y likely to be found?
1
views