(a) State whether or not the bonding in each substance is likely to be covalent: (i) glucose, (ii) nitrogen, (iii) aluminum hydroxide, (iv) ammonia, (v) neon.

Using Lewis symbols and Lewis structures, make a sketch of the formation of NCl3 from N and Cl atoms, showing valence-shell electrons. (a) How many valence electrons does N have initially? (c) How many valence electrons surround the N in the NCl3 molecule? (d) How many valence electrons surround each Cl in the NCl3 molecule?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Valence Electrons

Lewis Structures

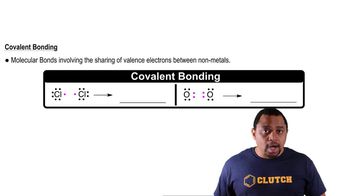
Covalent Bonding
(b) A substance, XY, formed from two different elements, melts at −33 °C. Is XY likely to be a covalent or an ionic substance?
Using Lewis symbols and Lewis structures, make a sketch of the formation of NCl3 from N and Cl atoms, showing valence-shell electrons. (b) How many bonds Cl has to make in order to achieve an octet?
Using Lewis symbols and Lewis structures, make a sketch of the formation of NCl3 from N and Cl atoms, showing valence-shell electrons. (e) How many lone pairs of electrons are in the NCl3 molecule?
Using Lewis symbols and Lewis structures, diagram the formation of PF3 from P and F atoms, showing valence-shell electrons. (a) How many valence electrons does P have initially? (c) How many valence electrons surround the P in the PF3 molecule? (d) How many valence electrons surround each P in the PF3 molecule?
