Textbook Question
An unknown gas is found to diffuse through a porous membrane 2.92 times more slowly than H2. What is the molecular weight of the gas? (a) 17.0 g/mol (b) 5.84 g/mol (c) 8.52 g/mol
3
views

 Verified step by step guidance
Verified step by step guidance

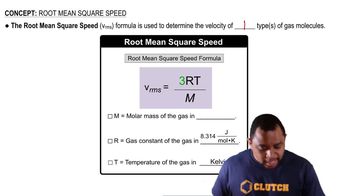
Two identical 732.0-L tanks each contain 212.0 g of gas at 293 K, with neon in one tank and nitrogen in the other. Based on the assumptions of kinetic–molecular theory, rank the gases from low to high for each of the following properties.
(a) Average speed
Two 112-L tanks are filled with gas at 330 K. One contains 5.00 mol of Kr, and the other contains 5.00 mol of O2. Considering the assumptions of kinetic–molecular theory, rank the gases from low to high for each of the following properties.
(c) Average speed