The average pH of normal arterial blood is 7.40. At normal body temperature 137 °C2, Kw = 2.4 * 10-14. Calculate 3H+4, 3OH-4, and pOH for blood at this temperature.
Ch.16 - Acid-Base Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 16, Problem 39b
Addition of the indicator methyl orange to an unknown solution leads to a yellow color. The addition of bromthymol blue to the same solution also leads to a yellow color. (b) What is the range (in whole numbers) of possible pH values for the solution?
 Verified step by step guidance
Verified step by step guidance1
Identify the pH range where methyl orange changes color. Methyl orange changes from red to yellow in the pH range of 3.1 to 4.4.
Identify the pH range where bromthymol blue changes color. Bromthymol blue changes from yellow to blue in the pH range of 6.0 to 7.6.
Since the solution turns yellow with both indicators, it must be in the range where both indicators show yellow.
Determine the overlapping pH range where both indicators show yellow. Methyl orange shows yellow above pH 4.4, and bromthymol blue shows yellow below pH 6.0.
Conclude that the pH of the solution is between 4.5 and 5.9, as this is the range where both indicators would show a yellow color.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is neutral, below 7 indicates acidity, and above 7 indicates basicity. The scale is logarithmic, meaning each whole number change represents a tenfold change in hydrogen ion concentration.
Recommended video:
Guided course

The pH Scale
Indicators and Their pH Ranges
Indicators are substances that change color in response to pH changes. Methyl orange transitions from red to yellow between pH 3.1 and 4.4, while bromthymol blue changes from yellow to blue between pH 6.0 and 7.6. The color change indicates the pH range of the solution based on the indicator used.
Recommended video:
Guided course
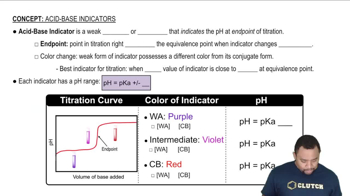
Acid-Base Indicators
Acid-Base Properties of Solutions
The acid-base properties of a solution determine its behavior with indicators. A solution that turns both methyl orange and bromthymol blue yellow suggests it is likely acidic but not strongly so, indicating a pH below 6.0. This information helps narrow down the possible pH range for the unknown solution.
Recommended video:
Guided course

Arrhenius Acids and Bases
Related Practice
Textbook Question
Textbook Question
Addition of phenolphthalein to an unknown colorless solution does not cause a color change. The addition of bromthymol blue to the same solution leads to a yellow color. (b) Which of the following can you establish about the solution: (i) A minimum pH, (ii) A maximum pH, or (iii) A specific range of pH values?
Textbook Question
Addition of phenolphthalein to an unknown colorless solution does not cause a color change. The addition of bromthymol blue to the same solution leads to a yellow color. (c) What other indicator or indicators would you want to use to determine the pH of the solution more precisely?
