Textbook Question
Use the Henderson–Hasselbalch equation to calculate the pH of each solution. c. a solution that contains 10.0 g of HC2H3O2 and 10.0 g of NaC2H3O2 in 150.0 mL of solution
1
views

 Verified step by step guidance
Verified step by step guidance


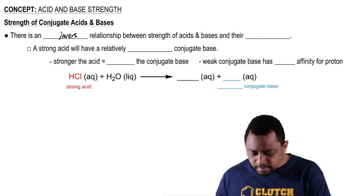
Use the Henderson–Hasselbalch equation to calculate the pH of each solution. c. a solution that contains 10.0 g of HC2H3O2 and 10.0 g of NaC2H3O2 in 150.0 mL of solution
Calculate the pH of the solution that results from each mixture. a. 50.0 mL of 0.15 M HCHO2 with 75.0 mL of 0.13 M NaCHO2
Calculate the pH of the solution that results from each mixture. b. 125.0 mL of 0.10 M NH3 with 250.0 mL of 0.10 M NH4Cl
Calculate the ratio of CH3NH2 to CH3NH3Cl concentration required to create a buffer with pH=10.34.
What mass of sodium benzoate should you add to 150.0 mL of a 0.15 M benzoic acid solution to obtain a buffer with a pH of 4.25? (Assume no volume change.)