Textbook Question
What is the maximum number of electrons that can occupy each of the following subshells? (a) 3s, (b) 2p, (c) 4d, (d) 5s.
1
views
1
rank

 Verified step by step guidance
Verified step by step guidance
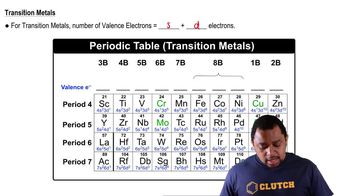
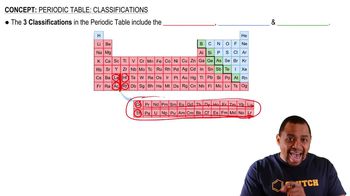
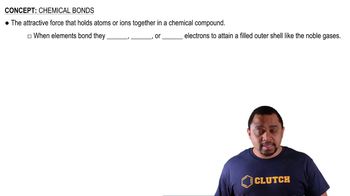
What is the maximum number of electrons that can occupy each of the following subshells? (a) 3s, (b) 2p, (c) 4d, (d) 5s.
(b) What are 'core electrons'?
(c) What does each box in an orbital diagram represent?
For each element, indicate the number of valence electrons, core electrons, and unpaired electrons in the ground state: (a) sodium (b) sulfur.