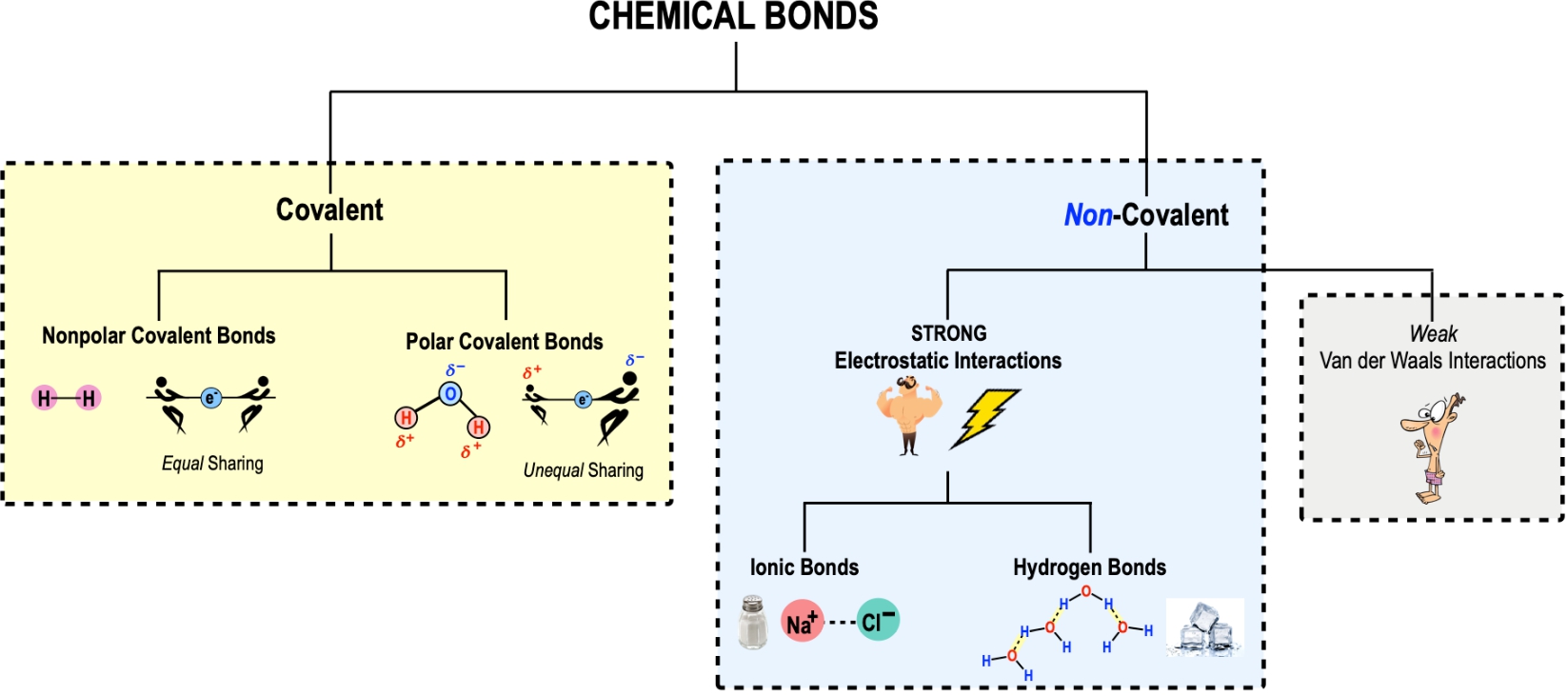
Chemical bonding is defined as the attractive forces between atoms that hold them together to form molecules and compounds. A molecule is any substance that contains two or more chemically bound atoms, which can be of the same or different elements. For instance, oxygen gas (O2) is a molecule because it consists of two oxygen atoms bonded together.
In contrast, compounds are a specific type of molecule that must contain at least two different elements. While all compounds are molecules, not all molecules qualify as compounds. For example, water (H2O) is a compound because it consists of hydrogen and oxygen, two different elements. On the other hand, O2 is a molecule but not a compound since it contains only one type of element.
Another example of a compound is glucose (C6H12O6), which contains carbon, hydrogen, and oxygen. This illustrates that compounds can be more complex, comprising multiple elements. Understanding the structure of compounds is essential, as they play crucial roles in biological systems, such as glucose being a primary energy source in living organisms.
The chemical formula is a concise way to represent the number and type of atoms in a molecule or compound. For glucose, the formula C6H12O6 indicates there are six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. This notation simplifies communication about chemical substances, making it easier to convey complex structures without drawing them out in detail. Similarly, H2O represents water, highlighting the utility of chemical formulas in chemistry.
As we progress, we will explore various types of chemical bonds and their significance in forming different molecules and compounds.